Reducing Late-Stage Diagnoses: How PanTum Detect Fits into Emerging MCED Strategies

Dr Letizia Gulino, Head of Science & Technology, RMDM Group
In recent decades, medical science has made tremendous progress in the early detection of certain cancers. However, the current approach remains fragmented and selective: while screening programs exist for breast, colorectal, and cervical cancers, the majority of malignancies are still detected only when symptoms arise, often too late for curative treatment.
This diagnostic gap has catalysed a shift toward a new model of cancer prevention: multi-cancer early detection (MCED) tests. These blood-based technologies analyse cell-free DNA (cfdna) or other tumour-derived biomarkers to detect multiple cancer types simultaneously from a single sample. The aim is to identify cancers before they become symptomatic, thus dramatically improving patient outcomes and reducing cancer-related mortality.
A new modelling study published in BMJ Open (Rous et al., 2025) provides compelling evidence to support this shift. Using a state-transition model calibrated with real-world data from the U.S. SEER cancer registry, the study simulated the effects of both annual and biennial MCED screening in individuals aged 50–79. The findings are striking: annual MCED screening reduced late-stage cancer diagnoses by 49% and cancer-related deaths within five years by 21%, compared to usual care. Even biennial screening led to a 39% reduction in late-stage diagnoses and 17% fewer deaths. Mortality reductions were most pronounced in cancers that currently lack organized screening programs—head and neck (79%), ovarian (71%), colorectal (66%), pancreatic (63%)—highlighting the potential of MCED to address current blind spots in public health. These numbers are not merely statistics—they represent lives saved, suffering avoided, and families spared the burden of late cancer diagnoses.
At RMDM Group, we find these findings not only encouraging but also validating. We believe the value of these findings must be contextualised within a broader technological and clinical framework. Our own platform, PanTum Detect, is a next-generation MCED test designed to align with—and exceed—the performance benchmarks outlined in the BMJ model.
Unlike most MCED solutions that focus primarily on fully developed malignancies, Pantum Detect demonstrates high sensitivity not only for early-stage cancers but also for precancerous lesions—a crucial feature that positions it not just as a diagnostic tool, but as a preventive instrument.
From Sensitivity to Specificity: Why Pantum Detect Stands Out
The BMJ Open model incorporated test characteristics such as sensitivity, stage-shift potential, and positive predictive value (PPV). For annual screening, a PPV of 43% was reported.
(see table1)
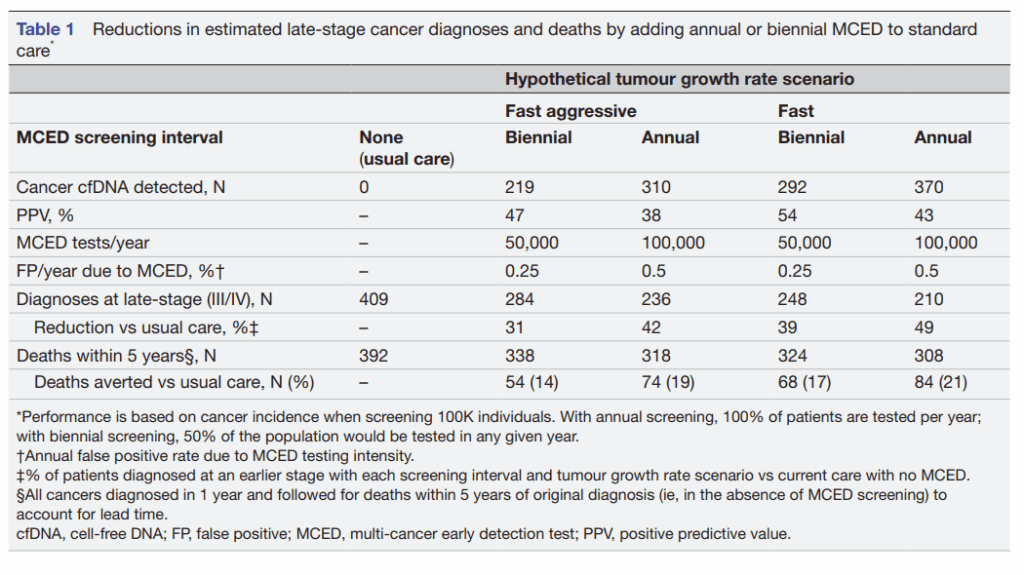 Rous B, et al. BMJ Open 2025;15:e086648. doi:10.1136/bmjopen-2024-086648
Rous B, et al. BMJ Open 2025;15:e086648. doi:10.1136/bmjopen-2024-086648
High sensitivity, when paired with low false-positive rates, is the gold standard in cancer screening. Pantum Detect excels on both fronts. In contrast to the average positive predictive value (PPV) reported in the BMJ Open study (43% for annual MCED screening), our internal validation data suggest a significantly higher PPV 66.47% due to Pantum’s refined detection algorithm and signal specificity.
This means fewer false positive, less anxiety for patients, and a reduced burden on healthcare systems in terms of unnecessary follow-up procedures. More importantly, the test’s minimal invasiveness and broad-spectrum detection capabilities make it a scalable solution for routine population-wide implementation.
The state-transition model presented by Rous et al. confirms that annual frequency ensures coverage for tumours with faster growth rates, and minimises the window for so-called “interval cancers,” and more frequent testing correlates with a higher rate of reduction in mortality.
A Complement, Not a Replacement
PanTum Detect is not intended to replace existing organ-specific screenings, but to complement them, filling the vast diagnostic blind spot that currently leaves too many cancers undetected until advanced stages. From pancreatic and ovarian to liver, oesophageal, and sarcomas. By detecting metabolic and immunological tumour signals in blood, PanTum Detect brings these previously elusive malignancies into the light early, affordably, and non-invasively.
A Vision for Preventive Oncology
As modelling studies like that of Rous et al. provide quantitative validation, and as technologies like PanTum Detect prove their readiness for clinical integration, we are clearly in a transition time from a reactive to a proactive model of oncology.
The question is no longer if MCED tests like Pantum Detect should be integrated into routine care, but how soon we can do so responsibly and at scale. The evidence is mounting, the technology is ready, and the potential is transformative. It is our responsibility as scientists, clinicians, and innovators to act on this momentum.
The future of oncology lies not in waiting for symptoms to appear, but in intercepting cancer in its silent beginnings. PanTum Detect represents this future, where precision prevention, not late-stage intervention, is the new standard.
Sources:
1. Assessment of the impact of multi-cancer early detection test screening intervals on late-stage cancer at diagnosis and mortality using a state-transition model
Brian Rous1, Christina A Clarke2, Earl Hubbell2, http://orcid.org/0000-0003-1509-8744Peter Sasieni3
Correspondence to Professor Peter Sasieni; p.sasieni@qmul.ac.uk
2. RMDM Group. Diagnostics. Prevention. Wellness. Available at: https://rmdm.group